Also see:
High Blood Pressure and Hypothyroidism
Sodium Deficiency and Stress
Sugar (Sucrose) Restrains the Stress Response
10 Tips for Better Sleep
Light is Right
Aldosterone, Sodium Deficiency, and Insulin Resistance
Low Sodium Diet: High FFA, Insulin Resistance, Atherosclerosis
Aldosterone as an endogenous cardiovascular toxin
Aldosterone and Thrombosis
Sodium Deficiency in Pre-eclampsia
Tryptophan Metabolism: Effects of Progesterone, Estrogen, and PUFA
Estrogen Increases Serotonin
Hypothyroidism and Serotonin
Omega -3 “Deficiency” Decreases Serotonin Producing Enzyme
Enzyme to Know: Tryptophan Hydroxylase
Role of Serotonin in Preeclampsia
Blood Pressure Management with Calcium & Dairy
Hypertension and Calcium Deficiency
Calcium Paradox
Calcium to Phosphorus Ratio, PTH, and Bone Health
Low CO2 in Hypothyroidism
High Estrogen and Heart Disease in Men
Thyroid Status and Cardiovascular Disease
Hypothyroidism and Shift in Death Patterns
A Cure for Heart Disease
Inflammatory TSH
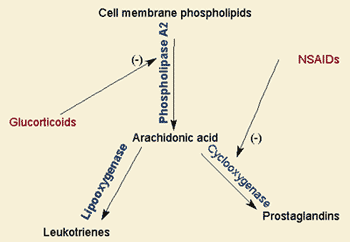
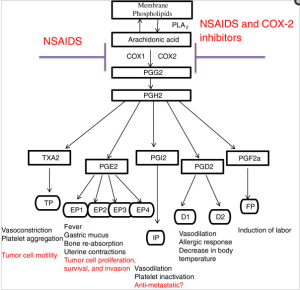
Unsaturated Fats and Heart Damage
“Normal” TSH: Marker for Increased Risk of Fatal Coronary Heart Disease
Thyroid Insufficiency. Is Thyroxine the Only Valuable Drug?
“Serotonin’s contribution to high blood pressure is well established. It activates the adrenal cortex both directly and through activation of the pituitary. It stimulates the production of both cortisol and aldosterone. It also activates aldosterone secretion by way of the renin-angiotensin system. Angiotensin is an important promoter of inflammation, and contributes to the degeneration of blood vessels with aging and stress. It can also promote estrogen production.”
“The loss of control over the water in the body is the result of energy failure, and hypertension is one of the adaptations that helps to preserve or restore energy production.”
“Stereotypes are important. When a very thin person with high blood pressure visits a doctor, hypothyroidism isn’t likely to be considered; even high TSH and very low T4 and T3 are likely to be ignored, because of the stereotypes. (And if those tests were in the healthy range, the person would be at risk for the “hyperthyroid” diagnosis.) But remembering some of the common adaptive reactions to a thyroid deficiency, the catabolic effects of high cortisol and the circulatory disturbance caused by high adrenaline should lead to doing some of the appropriate tests, instead of treating the person’s hypertension and “under nourished” condition.”
“Pregnant women sometimes develop very high blood pressure. In the 1950s, when new diuretics were being promoted by the drug companies, it became standard practice to give pregnant women diuretics and a low-salt diet to control their blood pressure. It should have been obvious (and it was obvious to people like Tom Brewer who thought physiologically, rather than mechanically) that the increase in pressure was the body’s response to an increased need for circulation. As the fetus grows, the blood volume must expand, to meet the increased circulatory needs of the uterus, placenta, and fetus. Two research projects showed that very large supplements of salt reliably normalized the high blood pressure in women with toxemia- of pregnancy. Other studies showed similar results with a supplement of progesterone. When the blood volume is able to expand as needed, circulation is adequate at normal pressure. When blood pressure is forced down by administering a diuretic to further diminish an already inadequate blood volume, the circulation of oxygen and nutrients to the fetus is seriously impaired, and a huge epidemic of mental retardation and hyperactivity, associated with low birthweight, began in the 1950s, and continued until eventually the fear of malpractice suits stopped the absurd practice.
A few years ago I asked a recently graduated physician what things he would want to consider in a patient with high blood pressure. Some of his suggestions for therapy were very reasonable, but his approach made it clear that he was thinking of circulation in a mechanical way, exactly as a skilled plumber would go about normalizing the circulation of water, without caring very much about what the water was being used for. The circulation of blood is nicely adjusted to meet the demands of the tissues. Blood pressure increases gradually with age, and individuals whose blood pressure stops increasing with age have been found to have a shorter life-expectancy than normal. Apparently, aged tissue is less efficient, and needs a needs a more strongly pumped stream of blood.”
“Most people are aware of some of the variations of bleeding and clotting that occur commonly. Bleeding gums, nose-bleeds, menstruation and its variations, and the spontaneous bruising (especially on the thighs) that many women have premenstrually, are familiar events that don’t seem to mean much to the medical world. Sometimes nose-bleeds are clearly stress-related, but the usual “explanation” for that association is that high blood pressure simply blows out weak blood vessels. Bleeding gums are sometimes stress related, but high blood pressure is seldom invoked to explain that problem.”
“Increased entry of calcium into cells is complexly related to increased exposure to unsaturated fatty acids, decreased energy, and lipid peroxidation. Osteoporosis, calcification of soft tissues and high blood pressure are promoted by multiple stresses, hypothyroidism, and magnesium deficiency. The particular direction a disease takes–diabetes, scleroderma, lupus, Alzheimer’s, stroke, etc.–probably results from the balance between resources and demands within a particular organ or system. Calcium overload of cells can’t be avoided by avoiding dietary calcium, because the bones provide a reservoir from which calcium is easily drawn during stress. (In fact, the reason calcium can temporarily help prevent muscle cramps.”
“Among physicians, toxemia (meaning poisons in the blood) has been used synonymously with preeclampsia, to refer to the syndrome in pregnant women of high blood pressure, albumin in the urine, and edema, sometimes ending in convulsions. Eclampsia is reserved for the convulsions themselves, and is restricted to the convulsions which follow preeclampsia, when there is “no other reason” for the seizure such as “epilepsy” or cerebral hemorrhage. Sometimes it is momentarily convenient to use medical terms, but we should never forget the quantity of outrageous ignorance that is attached to so many technical words when they suggest the identity of unlike things, and when they partition and isolate things which have meaning only as part of a process. Misleading terminology has certainly played an important role in retarding the understanding of the problems of pregnancy.”
“David McCarron has published a large amount of evidence showing how calcium deficiency contributes to high blood pressure. The chronic elevation of PTH caused by calcium deficiency causes the heart and blood vessels to retain calcium, making them unable to relax fully.”
“The ability to lower the cholesterol “risk factor” for heart attacks became a cultural icon, so that the contribution of estrogen and unsaturated oils to the pathologies of clotting could be ignored. Likewise, the contribution of unsaturated fats’ lipid peroxidation to the development of atherosclerotic plaques was simply ignored. But one of estrogen’s long established toxic effects, the reduction of tone in veins, was turned into something like a “negative risk factor”: The relaxation of blood vessels would prevent high blood pressure and its consequences, in this new upside down paradigm. This vein-dilating effect of estrogen has been seen to play a role in the development of varicose veins, in orthostatic hypotension, and in the formation of blood clots in the slow-moving blood in the large leg veins.”
“I think we can begin to see that the various “heart protective” ideas that have been promoted to the public for fifty years are coming to a dead end, and that a new look at the fundamental problems involved in heart disease would be appropriate. Basic principles that make heart disease more understandable will also be useful for understandingshock, edema, panic attacks, high altitude sickness, high blood pressure, kidney disease, some lung diseases, MS, multiple organ failure, and excitotoxicity or “programmed” cell death of the sort that causes degenerative nerve diseases and deterioration of other tissues.”
“In the 1950s, when the pharmaceutical industry was beginning topromote some new chemicals as diuretics to replace the traditional mercury compounds, Walter Kempner’s low-salt “rice diet” began to be discussed in the medical journals and other media. The diuretics were offered for treating high blood pressure, pulmonary edema, heart failure, “idiopathic edema,” orthostatic edema and obesity, and other forms of water retention, including pregnancy, and since they functioned by causing sodium to be excreted in the urine, their sale was accompanied by advising the patients to reduce their salt intake to make the diuretic more effective.”
“The hypo-osmolar blood of hypothyroidism, increasing the excitability of vascular endothelium and smooth muscle, is probably a mechanism contributing to the high blood pressure of hypothyroidism. The swelling produced in vascular endothelium by hypo-osmotic plasma causes these cells to take up fats, contributing to the development of atherosclerosis. The generalized leakiness affects all cells (see “Leakiness” newsletter), and can contribute to reduced blood volume, and problems such as orthostatic hypotension. The swollen endothelium is stickier, and this is suspected to support the metastasis of cancer cells. Inflammation-related proteins, including CRP, are increased by the hypothyroid hyperhydration. The heart muscle itself can swell, leading to congestive heart failure.”
“Pre-eclampsia and pregnancy toxemia have been corrected (Shanklin and Hodin, 1979) by both increased dietary protein and increased salt, which improve circulation, lower blood pressure, and prevent seizures, while reducing vascular leakiness. The effectiveness of increased salt in preeclampsia led me to suggest it for women with premenstrual edema, because both conditions typically involve high estrogen, hyponatremia, and a tendency toward hypo-osmolarity. Estrogen itself causes sodium loss, reduced osmolarity, and increased capillary leakiness. Combined with a high protein diet, eating a little extra salt usually helps to correct a variety of problems involving edema, poor circulation, and high blood pressure.
The danger of salt restriction in pregnancy has hardly been recognized by most physicians, and its danger inanalogous physiological situations is much farther from their consideration.”
“One of the things that happen when there isn’t enough sodium in the diet is that more aldosterone is synthesized. Aldosterone causes less sodium to be lost in the urine and sweat, but it achieves that at the expense of the increased loss of potassium, magnesium, and probably calcium. The loss of potassium leads to vasoconstriction, which contributes to heart and kidney failure and high blood pressure. The loss of magnesium contributes to vasoconstriction, inflammation, and bone loss. Magnesium deficiency is extremely common, but a little extra salt in the diet makes it easier to retain the magnesium in our foods.
Darkness and hypothyroidism both reduce the activity of cytochrome oxidase, making cells more susceptible to stress. A promoter of excitotoxicity, ouabain, or a lack of salt, can function as the equivalent of darkness, in resetting the biological rhythms (Zatz, 1989, 1991).”
“Both aldosterone and melatonin can contribute to the contraction of smooth muscle in blood vessels. Constriction of blood vessels in the kidneys helps to conserve water, which is adaptive if blood volume has been reduced because of a sodium deficiency. When blood vessels are inappropriately constricted, the blood pressure rises, while organs don’t receive as much blood circulation as they need. This impaired circulation seems to be what causes the kidney damage associated with high blood pressure, which can eventually lead to heart failure and multiple organ failure.”
“In hypothyroidism, thyrotropin-release hormone (TRH) is usually increased, increasing release of TSH. TRH itself can cause tachycardia, “palpitations,” high blood pressure, stasis of the intestine, increase of pressure in the eye, and hyperventilation with alkalosis. It can increase the release of norepinephrine, but in itself it acts very much like adrenalin. TRH stimulates prolactin release, and this can interfere with progesterone synthesis, which in itself affects heart function.”
“This is, to a great extent, the result of deliberate distortion by the drug industry of the issues involved. Beginning in the 1950s, the sale of new patented diuretics (replacing traditional diuretics) began in an atmosphere in which estrogen was being given to pregnant women, to prevent various complications of pregnancy (which in fact were caused by excessive estrogen). Excess estrogen as the cause of toxemia couldn’t be discussed openly, and the diuretics were sold as additional tools for controlling pregnancy-associated water retention, weight gain, high blood pressure, and damage to the fetus. Pregnant women were also told to diet to limit weight gain, and to sharply limit their consumption of salt. Estrogen, diuretics, salt restriction, and dieting were demonstrably all harmful to pregnant women and their babies, but they were imposed by the pharmaceutical medical establishment.”
“After a couple of years, I was satisfied that adequate protein and salt consistently prevented premenstrual edema. I read an article by some people who noticed that their patients who were on low sodium diets “for high blood pressure” very often developed insomnia. They knew that sodium restriction raised adrenalin levels, so they took their patients off the low sodium diet, and cured their insomnia.”
“Since vascular leakiness is involved in the inflammatory syndromes, as well as in shock, I
think it’s reasonable to treat them similarly, keeping in mind the importance of normal blood volume and viscosity. Sodium and sugar help to lower adrenaline, and so they can make a contribution to preventing high blood pressure, while maintaining normal hydration of the blood.”
“The old medical practice of restricting salt intake during pregnancy was an important factor
in causing it, so it’s interesting to look at the effects of salt restriction as a treatment for hypertension.
The pregnant woman’s blood volume expands, to permit the supply of energy to match the needs of the embryo. If the blood vohune doesn’t increase, or if it decreases, as in pregnancy toxemia, her blood pressure will increase. Typically, the decrease of blood volume is accompanied by an increase in the extracellular fluid, edema, resulting from leakage of fluid through the walls of the capillaries, and albumin appean; in the urine as it leaks through the capillaries in the kidneys. The amount of blood pumped by the heart, however, is increased in toxemia (Hamilton, 1952), showing that the increased blood pressure is at least partially compensating for the smaller volume of blood.
A similar situation, reduced blood volume and edema, can be seen (Tarazi, 1976) in “essential hypertension,” the “unexplained” high blood pressure that occurs more often with increasing age and obesity. At the beginning of “essential hypertension,” the amount of blood pumped is usually greater than normal.
In both situations, preeclampsia and essential hypertension. there is an increased amount of aldosterone, an adrenal steroid which allows the kidneys to retain sodium. and to lose potassium and ammonium instead. A restriction of salt in the diet causes more aldosterone to be produced, and increased salt in the diet causes aldosterone to decrease. One effect of aldosterone is to increase the production of a substance called vascular endothelial growth factor, VEGF, or vascular permeability factor, which causes capillaries to become leaky, and causes new blood vessels to grow.”
“The ability to lower the cholesterol “risk factor” for heart attacks became a cultural icon, so that the contribution of estrogen and unsaturated oils to the pathologies’-of clottIng could be ignored. Likewise, the contribution of unsaturated fats’ lipid peroxidation to the development of atherosclerotic plaques was simply ignored. But one of estrogen’s long established toxic effects, the reduction of tone in veins, was turned into something like a “negative risk factor”: The relaxation of blood vessels would prevent high blood pressure and its consequences, in this new upside down paradigm. This vein-dilating effect of estrogen has been seen to play a role in the development of varicose veins, in orthostatic hypotension; and in the formation of blood clots in the slow-moving blood in the large leg veins.”
“Originally, diabetes was understood to be a wasting disease, but as it became common for doctors to measure glucose, obese people were often found to have hyperglycemia, so the name diabetes has been extended to them, as type 2 diabetes. High blood sugar is often seen along with high blood pressure and obesity in Cushing’s syndrome, with excess cortisol, and these features are also used to define the newer metabolic syndrome.
Following the old reasoning about the sugar disease, the newer kind of obese diabetes is commonly blamed on eating too much sugar. Obesity, especially a fat waist, and all its associated health problems, are said by some doctors to be the result of eating too much sugar, especially fructose. (Starch is the only common carbohydrate that contains no fructose.) Obesity is associated not only with diabetes or insulin resistance, but also with atheroslcerosis and heart disease, high blood pressure, generalized inflammation, arthritis, depression, risk of dementia, and cancer.”
“In 1973 (in my book, Mind and Tissue) I reviewed old studies of cellular inhibition, which distinguished between the naturally quiet resting state of energized cells, and the state of protective inhibition, which prevents Injury or death from overstimutation and fatigue. In our “establishment physiology,” there has been no coherent theory on cellular inhibition, which means that cellular activity could hardly be understood correctly, either. Most of the facts are known, but they have seldom been put together in meaningful patterns, which would let us see that a few simple principles govern a great range of disturbing phenomena: seizures, shock, hypertension, fibrillation, cramps, restless legs, coma, insomnia, obsessive thinking, migraine, hyperactivity, even cell death and aging.”
“The interactions between estrogen and the polyunsaturated fats are now coming to be more
widely recognized as important factors in the inflammatory/hyperpermeable conditions that contribute to the development of heart and blood vessel disease, hypertension, cancer, autoimmune diseases, dementia, and other less common degenerative conditions.”
“Kidney disease, diabetes, pregnancy toxemia and retinal degeneration are probably the best
known problems involving vascular leakage, but increasingly, cancer and heart disease are being recognized as consequences of prolonged permeability defects. Congestive heart failure and pulmonary hypertension commonly cause leakage of fluid into the lungs, and shock of any sort causes the lung to get “wet,” a waterlogged condition called “shock lung.” Simply hyperventilating for a couple of minutes will increase leakage from the blood into the lungs; hyperventilation decreases carbon dioxide, and increases serotonin and histamine.”
“The use of antiserotonin drugs in alleviating stroke, hypertension, heart failure, diabetes, depression, obesity, rheumatoid arthritis, lupus (SLE), fibrosis, wheezing, and migraine suggests the importance of hyperserotonemia in causing disease.”
“Preeclampsia, or a syndrome of pregnancy induced hypertension, occurs in about 10% of
pregnancies, and it’s the main cause of maternal death and sickness of the newborn.
Thomas Brewer, about 50 years ago, made it clear that a protein deficiency is the main cause of preeclampsia. Protein deficiency causes a general inflammatory condition, with increased serotonin:”
“Aging, shock, hypertension, cancer, heart failure, and many other biological problems involve edema as a central factor, and relieving the edema is probably an essential part of solving those problems.”
“Shock, pneumonia, and hypertension are among the consequences of edema. Understanding edema is essential for understanding stress, for preventing stress induced sickness, and for resuscitation.”
“In the premenstrual syndrome, as in pregnancy, a progesterone deficiency can cause
generalized edema. Tom Brewer, who founded the Society for the Protection of the Unborn
through Nutrion, and S. Shanklin and J. Hodin, in Maternal Nutrition and Child Health, argued that salt restriction, expecially when combined with diuretics and a diet without adequate protein, caused exaggerated edema in pregnancy, producing a great risk of hypertension by reducing the blood volume needed for adequate perfusion of the kidneys, and damaged the development of the fetus, because of inadequate blood perfusion of the uterus and placenta.”
“Recently, a British physician, from Mongolia or northern China, studied the incidence of hypertension in her native region, where people consume at least 30 grams of salt per day. She found no hypertension at all, even among the oldest people. In my experiments, it has taken the body only two or three days to adjust completely to a massive change in salt consumption. Many hormones adjust quickly to retain or release sodium, according to the amount consumed, if the person is otherwise well nourished. Hypothyroid people, however, are unable to maintain a normal sodium concentration in their body fluids even when they increase their salt consumption.”
“Thyroid, progesterone, protein, and salt are powerful defenses against all sorts of stress associated symptoms, including hot flashes, insomnia, cramps, PMS, edema, toxemia of pregnancy, low-birth-weight babies, epilepsy, heart diseases, hypertension, strokes, migraine, inflammatory diseases hypoglycemia, fatigue and depression. The first approach to an appropriate diet would be to use at least a quart of milk and a quart of orange juice daily, well salted chicken broth, and frequent snacks, especially salty foods.”
“The “metabolic syndrome,” that involves diabetes, hypertension, and obesity, is associated
with high PTH (Ahlstrom, et al., 2009; Hjelmesaeth, et al., 2009).”
“Substances such as PTH, nitric oxide, serotonin, cortisol, aldosterone, estrogen, thyroid stimulating hormone, and prolactin have regulatory and adaptive functions that are essential, but that ideally should act only intermittently, producing changes that are needed momentarily. When the environment is too stressful, or when nutrition isn’t adequate, the organism may be unable to mobilize the opposing and complementary substances to stop their actions. In those situations, it can be therapeutic to use some of the nutrients as supplements. Calcium carbonate (eggshell or oyster shell, for example) and vitamins D and K, can sometimes produce quick antistress effects, alleviating insomnia, hypertension, edema, inflammations and allergies, etc., but the regular use of milk and cheese can prevent many chronic stress-related diseases.”
“One of the oldest tests for hypothyroidism was the Achilles tendon reflex test, in which the rate
of relaxation of the calf muscle corresponded to thyroid function–the relaxation is slow in hypothyroid people. Water, sodium and calcium are more slowly expelled by the hypothyroid muscle. Exactly the same slow relaxation occurs in the hypothyroid heart muscle, contributing to congestive heart failure, because the semi-contracted heart can’t receive as much blood as the normally relaxed heart. The hypothyroid blood vessels are unable to relax properly, contributing to hypertension. Hypothyroid nerves don’t easily return to their energized relaxed state, leading to insomnia, paresthesias, movement disorders, and nerves that are swollen and very susceptible to pressure damage.”
“Premenstrual syndrome, preeclampsia or pregnancy hypertension, congestive heart failure, brain swelling and seizures all involve disturbances of salt and water regulation, but the mechanical medical tradition has almost always substituted beliefs for facts.
Because of beliefs about cell physiology, most medical publications have argued for sodium
restriction in those situations, but the evidence is clear that inadequate salt retention is usually their outstanding pathological feature.
Progesterone has been an effective treatment in all of those conditions, and it increases the ability of the kidneys to retain sodium.”
“Instead of considering the significance of sodium’s effects on albumin, aldosterone. and
VEGF, textbooks have often talked about the factors that “pump” sodium, and factors that specifically regulate the movement of water. Experiments in which an excess of aldosterone is combined with a high salt intake produce increased blood pressure, and-by invoking various genes-salt is said to cause hypertension in certain people. This reasoning is hardly different from the reasoning of the drug companies in the 19505 who said that since women with toxemia have hypertension and edema, they should be treated with a diuretic and a low saIt diet, to eliminate water and to reduce blood pressure.”
“The increase of adrenalin, caused by a deficiency of sodium, is one ofthe factors that can
increase blood pressure; if the tissues’s glycogen stores are depleted, the adreoaJin will mobilize free fatty acids from the tissues, which tends to inhibit energy production from glucose, and to increase leakiness. After I had read Tom Brewer’s work on preventing or curing preeclampsia with added salt I realized that the premenstrual syndrome involved some of the features of preeclampsia (edema, insomnia, cramps, hypertension, salt craving), so I suggested to a friend that she might try salting her food to taste, instead of trying to restrict salt to “prevent edema.” She immediately noticed that it prevented her monthly edema problem. For several years, all the women who tried it had similarly good results, and often mentioned that their sleep improved. I mentioned this to several people with sleep problems, and regardless of age, their sleep improved when they ate as much salt as they wanted. Around that time, several studies had shown that salt restriction increases adrenalin, and one study showed that most old people on a low sodium diet suffered from insomnia, and had unusually high adrenalin. When they ate a normal amount of salt their adrenalin was normalized,and they slept better.”
“Even before aldosterone was identified, progesterone’s role in regulating the salts, water, and energy metabolism was known, and after the functions of aldosterone were identified, progesterone was found to protect against its harmful effects, as it protects against an excess of cortisol, estrogen, or the androgens. New anti-aldosterone drugs are available that are effective for treating hypertension and heart failure, and their similarity to progesterone is recognized.”
“After 40 years the medical profession quietly retreated from their catastrophic approach to pregnancy toxemia, but in the more general problem of essential hypertension, the mistaken ideology is being preserved, even as less hannfu1 treatments are introduced. That ideology prevents a comprehensive and rational approach to the prohlems of stress and aging.”
“In what follows, I am acting as though the doctrines of genetic determination and regulation by membranes were mere historical relics. The emerging control systems are now clear enough that we can begin to use them to reverse the degenerative diseases: Alzheimer’s dementia, epileptic dementia, arthritis, osteoporosis, depression, hypertension, hardening of the heart and blood vessels, diabetes, and some types of tumor, immunodeficiencies, reflex problems, and special atrophic problems, including clearing of amyloid and mucoid deposits. I think many people experience regenerative age-regressing when many circumstances are just right; for example, taking a trip to the mountains in the spring with friends can optimize several basic regulatory systems.”
“”All cell death is characterized by an increase of intracellular calcium….” “Increase of cytoplasmic free calcium may therefore be called ‘the final common path’ of cell disease and cell death. Aging as a background of diseases is also characterized by an increase of intracellular calcium. Diseases typically associated with aging include hypertension, arteriosclerosis, diabetes mellitus and dementia.”
T. Fujita, “Calcium, parathyroids and aging,” in Calcium-Regulating
Hormones. 1. Role in Disease and Aging, H. Morii, editor, Contrib.
Nephrol. Basel, Karger, 1991, vol. 90, pp. 206-211.”
“In 1933 James Shute was recommending the use of vitamin E for preventing the clotting problems associated with pregnancy, that often lead to miscarriage. He based his work on animal studies, that led to vitamin E’s being known as the “fertility vitamin.” Later, his sons Wilfred and Evan reported that vitamin E could prevent heart attacks, birth defects, complications of diabetes, phlebitis, hypertension, and some neurological problems.”
“About 25 years ago, David McCarron noticed that the governments data on diet and hypertension showed that the people who ate the most salt had the lowest blood pressure, and those who ate the least salt had the highest pressure. He showed that a calcium deficiency, rather than a sodium excess, was the most likely nutritional explanation for hypertension.”
“The antimetabolic actions of PTH mimic those seen in aging and diabetes, and surgical removal of the parathyroid glands has been known to eliminate diabetes. PTH can cause diuresis, leading to loss of blood volume and dehydration, hypertension, paralysis, increased rate of cell division, and growth of cartilage, bone, and other tissues.”
“Although I started this newsletter with the thought of discussing the Mead acids–the unsaturated (n-9) fats that are formed under certain conditions, especially when the dietary polyunsaturated fatty acids are “deficient”–and their prostaglandin derivatives as a distinct anti-stress, anti-aging system, the loss of which makes us highly susceptible to injury, I will save that argument for a future time, leaving this newsletter as an addition to the view that an excess of the polyunsaturated fats is central to the development of degenerative diseases: Cancer, heart disease, arthritis, immunodeficiency, diabetes, hypertension, osteoporosis, connective tissue disease, and calcification.”
“If the physiology of shock has some relevance for eclampsia, so does the physiology of heart failure, since Meerson has shown that it is a consequence of uncompensated stress. The failing heart shifts from mainly glucose oxidation to the inefficient use of fatty acids, which are mobilized during stress, and with its decreased energy supply, it is unable to beat efficiently, since it remains in a partly contracted state. Estrogen (which is increased in men who have had heart attacks) is another factor which decreases the heart’s stroke volume, and estrogen is closely associated with the physiology of the free unsaturated fatty acids. The partly contracted state of the heart is effectively a continuation of the partly contracted state of the blood vessels that causes the hypertension, and reduced tissue perfusion seen in shock and eclampsia.”
“A diet low in sodium and protein probably kills many more people than has been documented. If old age is commonly a hypovolemic condition, then the common salt restriction for old-age hypertension is just as irrational as is salt-restriction in pregnancy or in shock. Thyroid (T 3), glucose, sodium, magnesium and protein should be considered in any state in which weakened homeostatic control of the composition of plasma is evident.”
“Tom Brewer demonstrated the importance of eating enough salt during pregnancy, to maintain adequate blood volume. When salt is restricted during pregnancy, the inadequate blood volume doesn’t carry enough oxygen and food to the uterus to allow full development of the baby, and the kidneys secrete a hormone to increase the circulation, creating a tendency toward high blood pressure. Following Brewer’s research, I saw that extra sodium should help in other situations involving circulatory inefficiency. Premenstrual edema, insomnia, and even high blood pressure often respond very well to a little extra sodium in the diet. One of the most important effects of sodium is that it tends to spare magnesium. which is likely to be lost during stress and hypothyroidism. If we eat salty foods when we crave them, we are able to retain our magnesium more easily. Sodium also helps to regulate blood sugar, for example by improving its absorption from the intestine. There is even evidence that sodium can spare protein, since, if there isn’t enough sodium to excrete into the urine to balance acids, the kidneys will waste protein to produce ammonium as an ionic substitute for sodium. But I think the most important point to remember is that it is essential for mainting adequate blood volume, and that it is almost always unphysiological and irrational to restrict sodium intake, because reduced blood volume tends to reduce the delivery of oxygen and nutrients to all tissues, leading to many problems. The emotional tension many people feel when they crave salt is, in some cases, the result of increased adrenaIin, reflecting a real biological problem.”
“Part of the reason for the common medical disbelief in the efficacy of vitamin E is that it doesn’t work like a drug – a big dose doesn’t immediately force the blood pressure down. Sometimes, in fact, the first effect is to strengthen a damaged heart, raising the blood pressure for a few days. But it does eventually remove many of the causes of high blood pressure, and I have never seen it fail to lower high blood pressure.”
“Other vitamins that can improve circulation by opening the small blood vessels are folic acid and niacin. Vitamin C can help to eliminate toxins that could contribute to high blood pressure.”
“The fact that estrogen , acting as an antagonist to vitamin E, could promote high blood pressure had distracted my attention from the opposite effect, being produced by an antagonism to thyroxin.”
“According to Barnes, nearly all hypertension can be helped with thyroid.”
“Since thyroxine potentiates adrenalin, which maintains blood pressure, excess’ estrogen. antagonizing thyroid, could tend to lower blood pressure through this system . The thermogenic effect of progesterone might act by way of thyroxin: if so, it might be the best way of counteracting estrogen and promoting thyroxin activity. The opposite reaction to high estrogen-low thyroid is also known to occur: an elevation of brain catecholamines and also an elevation of blood pressure. Thus a thyroid supplement can often correct hypertension, as well as hypotension.”