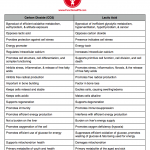
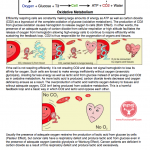
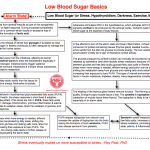
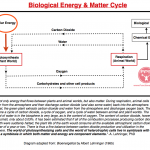
Also see:
Estrogen Increases Serotonin
Anti Serotonin, Pro Libido
Gelatin > Whey
Thyroid peroxidase activity is inhibited by amino acids
Whey, Tryptophan, & Serotonin
Tryptophan, Fatigue, Training, and Performance
Carbohydrate Lowers Free Tryptophan
Protective Glycine
Intestinal Serotonin and Bone Loss
Hypothyroidism and Serotonin
Gelatin, Glycine, and Metabolism
Whey, Tryptophan, & Serotonin
Tryptophan, Sleep, and Depression
Carbohydrate Lowers Serotonin from Exercise
Inflammatory TSH
Antihistamines and some of the antiserotonin drugs (including “dopaminergic” lisuride and bromocriptine) are sometimes useful in cancer treatment, but the safe way to lower serotonin is to reduce the consumption of tryptophan, and to avoid excessive cortisol production (which mobilizes tryptophan from the muscles). Pregnenolone and sucrose tend to prevent over-production of cortisol. -Ray Peat, PhD
Neuro Endocrinol Lett. 2000;21(5):405-408.
Efficacy of bromocriptine in the treatment of metastatic breast cancer- and prostate cancer-related hyperprolactinemia.
Lissoni P, Mandalà M, Giani L, Malugani F, Secondino S, Zonato S, Rocco F, Gardani G.
OBJECTIVE:
Hyperprolactinemia is a frequent evidence occurring in both metastatic breast cancer and prostate cancer, and it has been proven to be associated with poor prognosis and reduced efficacy of the anticancer therapies. Therefore, the pharmacological control of cancer-related hyperprolactinemia could improve the prognosis of advanced breast and prostate carcinomas. Unfortunately, at present it is still controversial which may be the treatment of cancer-related hyperprolactinemia, which could depend at least in part on a direct autocrine production by cancer cells themselves. The present study was performed to evaluate the acute effects of the long-acting dopaminergic agonist bromocriptine on cancer-related hyperprolactinemia.
METHODS:
The study included 10 women affected by metastatic breast cancer and 10 men with metastatic prostate cancer, showing persistent hyperprolactinemia. Venous blood samples were collected before bromocriptine, and 2, 4, 10 and 24 hours after bromocriptine administration (2.5 mg orally) serum levels of PRL were measured with the double antibody RIA method.
RESULTS:
Bromocriptine induced a normalization of PRL levels in both groups of patients with breast and prostate cancers. Moreover, mean levels of PRL persisted significantly lower than those found before therapy during the whole 24-hour circadian period.
DISCUSSION:
This preliminary study shows that low-dose bromocriptine is sufficient to acutely normalize PRL secretion in both metastatic breast cancer and prostate carcinoma patients, irrespectively of the mechanisms involved in inducing cancer-related hyperprolactinemia. Therefore, low-dose bromocriptine could be recommended in association with the classical antitumor therapies in the treatment of metastatic breast cancer and prostate carcinoma patients showing cancer-related hyperprolactinemia, in an attempt to improve the efficacy of anticancer therapies themselves.
The Lancet, Volume 331, Issue 8586, Pages 609 – 610, 19 March 1988
PERIOPERATIVE BROMOCRIPTINE ADJUVANT TREATMENT FOR OPERABLE BREAST CANCER
I.S. Fentiman , M.A. Chaudary , D.Y. Wang , K. Brame , R.S. Camplhjohn , R.R. Millis
Blood levels of prolactin are consistently raised in women who have undergone mastectomy for breast cancer, probably because of the stress of surgery. Since this increase in concentration of a known breast epithelial growth promoter might stimulate proliferation in micrometastatic cells shed at the time of surgery, a pilot study was conducted to try to abolish this effect. 38 patients with suspected operable breast cancer were given either bromocriptine (18) or placebo (20) tablets for 5 days preoperatively and thereafter for 3-10 days. Bromocriptine-treated patients showed significant reductions in prolactin levels and in S-phase fraction of tumour cells within the primary infiltrating carcinoma. There were no major side-effects. Perioperative bromocriptine may provide another approach to adjuvant therapy, possibly combined with endocrine or cytotoxic treatment for patients with axillary nodal metastases.
The Lancet, Volume 314, Issue 8133, Pages 66 – 69, 14 July 1979
REDUCTION OF PITUITARY-TUMOUR SIZE IN PATIENTS WITH PROLACTINOMAS AND ACROMEGALY TREATED WITH BROMOCRIPTINE WITH OR WITHOUT RADIOTHERAPY
J.A.H. Wass , M.O. Thorner 1, M. Charlesworth , P.J.A. Moult , J.E. Dacie , A.E. Jones , G.M. Besser
69 patients with prolactin-secreting or growth-hormone-secreting pituitary tumours were treated with bromocriptine with or without pituitary irradiation and followed up for 6 months to 6 1/2 years. Of 26 patients with prolactinomas, 11 had external pituitary irradiation in addition to bromocriptine. There was evidence of shrinkage of the pituitary tumour (either a reduction in fossa size or loss of visual-field defects) in 6 of these patients (23%), 3 of whom had been treated with bromocriptine alone. Of 43 acromegalic patients, 30 received external pituitary irradiation. 8 (19%) showed evidence of shrinkage of the pituitary tumour, including 2 who had received no radiotherapy. 1 patient treated with bromocriptine alone showed striking reduction in the size of his suprasellar extension, as assessed by serial computed-tomography scans over 11 months. At the same time his visual-field defects resolved and his deficient corticotrophin and thyrotrophin reserves returned to normal. Bromocriptine can reduce the size of both prolactin-secreting and growth-hormone-secreting pituitary tumours, and this is of potential importance in their management.
The Lancet, Volume 319, Issue 8266, Pages 245 – 249, 30 January 1982
HYPERPROLACTINAEMIA IN MEN—RESPONSE TO BROMOCRIPTINE THERAPY
R.W.G. Prescott a b, P. Kendall-Taylor a b, K. Hall a b, D.G. Johnston a b, A. Crombie a b, A. Mcgregor a b, K. Hall a b
Men with hyperprolactinaemia present with large tumours. Conventional therapy with surgery and/or irradiation is unsatisfactory, with up to 100% of patients remaining hyperprolactinaemic (or subsequently developing pituitary insufficiency). In view of reports of bromocriptine-induced regression of prolactinomas, eight consecutive male hyperprolactinaemic patients with impotence and/or symptoms related to local tumour effects were treated with bromocriptine 20 mg daily as sole therapy for 3-11 months. Symptoms were relieved partly or completely in seven patients and serum prolactin was restored to normal or near normal in all men. Serum thyroxine and plasma cortisol response to hypoglycaemia became normal in two men who had subnormal values before therapy. Mean serum growth hormone response to hypoglycaemia rose significantly as did plasma testosterone concentrations. Evidence of tumour regression, sometimes massive, was seen in the six patients who underwent repeat radiology. The symptomatic relief and biochemical and radiological improvement in these patients indicate that bromocriptine therapy may now be the treatment of choice for hyperprolactinaemic men with large tumours.
The Lancet, Volume 324, Issue 8396, Pages 187 – 192, 28 July 1984
EFFECT OF DOPAMINE AGONIST WITHDRAWAL AFTER LONG-TERM THERAPY IN PROLACTINOMAS
D.G. Johnston , P.Kendall Taylor , M. Watson , K. Hall , D. Patrick , D.B. Cook
The clinical, radiological, and biochemical effects of dopamine agonist withdrawal after long-term treatment were investigated in seven women and eight men who had been treated for prolactinomas for 1·5 to 7 (mean 3·7) years. Before treatment, serum prolactin concentrations were 1473 to 115 000 mU/1, all patients had abnormal radiological findings, and six had suprasellar extensions of pituitary tumours. Treatment with either bromocriptine or pergolide relieved symptoms and suppressed prolactin secretion in most patients. The size of the residual tumour was defined by doing fourth generation computerised . tomographic scans immediately before termination of therapy, and evidence of tumour re-expansion was sought on scans repeated 5-39 weeks later. After discontinuation of treatment, symptoms recurred in 13 of 15 patients and hyper-prolactinaemia redeveloped in 14. Other pituitary function tests remained unchanged or improved. In 13 of 15 patients tumour or gland size did not change after withdrawal of treatment. One man had a marginal increase in tumour size, while in another the pituitary tumour shrank. Thus, although cessation of long-term dopamine agonist therapy leads to recurrence of symptoms and hyperprolactinaemia, rapid tumour regrowth is uncommon and of small extent, and other pituitary function is not altered in the short term.
Br Med J. 1979 September 22; 2(6192): 700–703.
Effects of bromocriptine on pituitary tumour size.
A M McGregor, M F Scanlon, R Hall, and K Hall
In a prospective study designed to assess the influence of bromocriptine on pituitary tumour size 12 patients with pituitary tumours, eight of whom had suprasellar extensions, were treated for three months with 20 mg of bromocriptine daily after a gradual increase to this dose. The group comprised eight women and four men, five with prolactin-secreting adenomas, four with acromegaly, two with functionless adenomas, and one with Nelson’s syndrome. All five patients with prolactin-secreting adenomas showed a reduction in pituitary tumour size as assessed by computerised tomography and metrizamide cisternography accompanied by a fall in prolactin concentrations and clinical and biochemical improvement in their hypopituitarism. One patient in this group had a visual-field defect before treatment, and this resolved. There was no radiological evidence of reduction in tumour size in the remaining seven patients, though this might refect the fairly short duration of treatment, particularly in view of the ancillary evidence of clinical, biochemical, and visual-field improvement in some of the patients. These results emphasise the potential value of bromocriptine in treating patients with large prolactinomas or recurrences of such tumours after previous chiasmal decompression and conventional external megavoltage irradiation on the pituitary.
Br Med J (Clin Res Ed). 1982 June 26; 284(6333): 1908–1911.
Bromocriptine in management of large pituitary tumours.
J A Wass, J Williams, M Charlesworth, D P Kingsley, A M Halliday, I Doniach, L H Rees, W I McDonald, and G M Besser
Bromocriptine has an accepted place in the management of small pituitary tumours that secrete either prolactin or growth hormone. The treatment of large tumours with extrasellar extensions is more difficult, however: though surgery is the standard treatment, it is often unsuccessful in returning excessive hormone secretion to normal and may cause hypopituitarism. A prospective trial was undertaken to assess the frequency with which changes in pituitary function and size of large tumours occurs. Nineteen patients were studied before and during treatment with bromocriptine (7.5 to 60 ml/day) for three to 22 months, using contrast radiology and a detailed assessment of pituitary function. Eighteen patients had hyperprolactinaemia and two of these also had raised concentrations of growth hormones; one patient had an apparently non-functioning tumour. In 12 patients (63%) tumour size decreased with bromocriptine and no tumour enlarged. Nine patients had visual-field defects, which improved in seven, becoming normal in five. Pituitary function improved in nine patients (47%) becoming entirely normal in three. Bromocriptine should be the treatment of choice in patients with large pituitary tumours with extrasellar extensions, provided close supervision is maintained.
JAMA. 1982 Jan 15;247(3):311-6.
Bromocriptine reduces pituitary tumor size and hypersection. Requiem for pituitary surgery?
Spark RF, Baker R, Bienfang DC, Bergland R.
Twelve patients with pituitary tumor whose prior treatment included surgery and radiotherapy in four, surgery alone in four, radiotherapy alone in one, and none in three were studied. Nine had hyperprolactinemia, two had elevated serum growth hormones, and one had no pituitary hormone excess. Visual field defects were present in six. All had pituitary-gonadal insufficiency manifested as impotence or amenorrhea. All were tested with bromocriptine, 7.5 to 25 mg daily, and followed up for eight to 27 (mean 15) months. Serum prolactin levels decreased to normal in seven of nine patients. Serum growth hormone values were normalized in both acromegalics. When hormone levels were reduced to normal, pituitary tumor size decreased. Vision was restored to normal in five of six patients, including one patient with pituitary tumor but no pituitary hormone excess. Bromocriptine corrects the physiological defects associated with pituitary tumors that have been incompletely treated with surgery, radiotherapy, or both and may be a useful primary treatment for patients with pituitary tumors.
Am J Med. 1983 Nov;75(5):868-74.
Hyperprolactinemia. Long-term effects of bromocriptine.
Johnston DG, Prescott RW, Kendall-Taylor P, Hall K, Crombie AL, Hall R, McGregor A, Watson MJ, Cook DB.
Patients with hyperprolactinemia may be managed by pituitary surgery or irradiation, bromocriptine treatment, or a combination of these methods, and some patients remain untreated. Little is known of the long-term consequences of some of these therapeutic regimens. Forty-six hyperprolactinemic patients (40 female and six male) managed solely with bromocriptine or no treatment over a period of 12 months to six years were therefore evaluated in this study. Nine patients with radiologically normal pituitary fossae were untreated and 10 received bromocriptine, 7.5 to 10 mg daily, while 20 patients with radiologic evidence of a pituitary tumor were treated with bromocriptine, generally 10 to 20 mg daily. Patients were assessed clinically, biochemically, and radiologically before treatment and at least six weeks after discontinuation of therapy. A further seven patients were similarly assessed before and after eight bromocriptine-induced pregnancies. Symptoms persisted in the untreated group of nine patients, although menstruation returned in four of the females with previous amenorrhea; serum prolactin levels remained elevated, other pituitary function did not change, and pituitary fossae remained normal radiologically. In all patients treated with bromocriptine, symptoms improved irrespective of radiologic findings on the pituitary, and were abolished in 67 percent during treatment associated with a decrease in serum prolactin levels in all, and a return of levels to within normal limits in 80 percent of patients. Persistent side effects were usually dose-related, but remained troublesome in 13 percent. Bromocriptine-induced tumor regression was evident radiologically in all patients with suprasellar tumor tissue and in some with purely intrasellar adenomas. This effect occurred rapidly and persisted or increased throughout follow-up. On discontinuation of treatment, prolactin levels remained significantly lower than before therapy (mean 2,934 versus 5,052 mU/liter, p less than 0.05) but were within the normal range in only two patients. Other pituitary function was unaltered, or improved in some patients with definite tumors. Bromocriptine-induced pregnancy produced no permanent change in clinical, biochemical, or radiologic status. Long-term bromocriptine treatment for hyperprolactinemia is thus highly effective in alleviating symptoms and suppressing prolactin secretion, and induces persistent tumor regression on treatment without deterioration of other pituitary function in patients with macroadenomas. On discontinuation of therapy, however, hyperprolactinemia usually recurs, and treatment may therefore need to be continued for years.
Am J Dis Child. 1993 Oct;147(10):1057-61.
Prolactin-secreting macroadenomas in adolescents. Response to bromocriptine therapy.
Tyson D, Reggiardo D, Sklar C, David R.
OBJECTIVE:
To report five cases of prolactin (PRL)-secreting macroadenomas in adolescents, including their presentations and responses to bromocriptine mesylate treatment.
PATIENTS:
Five adolescents (three females and two males) aged between 12.5 and 17 years were diagnosed as having PRL-secreting macroadenomas at the pediatric endocrine service at New York University Medical Center between 1987 and 1989. Presenting complaints included visual field deficits, gynecomastia, and amenorrhea, both primary and secondary. All patients demonstrated some feature of hypogonadism or pubertal arrest. Diagnostic criteria included an elevated serum PRL level (mean, 1670 micrograms/L; range, 610 to 3700 micrograms/L) and visualization of a pituitary tumor that measured greater than 1 cm by either a computed tomographic scan or magnetic resonance imaging (mean size, 2.7 cm; range, 1.4 to 4 cm).
INTERVENTIONS:
Each patient was treated with bromocriptine mesylate at an oral dose of 7.5 mg/d. The patients continued with that treatment for the duration of the study period.
MEASUREMENTS AND RESULTS:
Anterior pituitary function was evaluated in four of five patients before treatment. All four were growth hormone deficient. Three patients were also gonadotropin deficient. Thyrotropin (thyroid-stimulating hormone) and corticotropin (adrenocorticotropic hormone) deficiencies were demonstrated in three patients who had multiple pituitary deficits. Follow-up testing included serial PRL measurements and radiographic imaging of tumor size. All patients demonstrated a marked decrease in PRL levels, as well as in tumor size (mean shrinkage, 70%). The three patients who initially had visual field deficits showed significant improvement of vision with bromocriptine therapy. Follow-up study of anterior pituitary function showed significant improvement with bromocriptine treatment in three patients.
CONCLUSIONS:
Bromocriptine was quite effective in the shrinkage of PRL-secreting macroadenomas in all our patients. It is a noninvasive treatment that can preserve and restore vision, as well as pituitary function, which is integral to continued growth and sexual maturation of the adolescent. Bromocriptine is preferable to surgery or radiation in the treatment of PRL-secreting macroadenomas in the adolescent.
Ups J Med Sci. 1982;87(3):259-67.
Rapid regression of pituitary tumours during bromocriptine treatment of women with hyperprolactinaemia.
Bergh T, Nillius SJ, Lundberg PO, Moström U, Enoksson P, Ohman L, Wide L.
Four hyperprolactinaemic women with large pituitary adenomas with suprasellar extension were given primary tumour therapy with bromocriptine. The treatment resulted in rapid tumour regression in all the women, as verified by repeated computerized tomography (CT) scans. Pronounced visual field defects were present in three of the four women before treatment. All of them had marked improvement of vision within a few days after the initiation of bromocriptine therapy and they regained normal or nearly normal visual fields during the treatment. The raised serum prolactin concentrations decreased to normal levels in all the women. Thus, medical treatment with bromocriptine can induce rapid tumour regression in patients with hyperprolactinaemia and large pituitary tumours.
Obstet Gynecol. 1989 Mar;73(3 Pt 2):517-20.
Successful treatment of a prolactin-producing pituitary macroadenoma with intravaginal bromocriptine mesylate: a novel approach to intolerance of oral therapy.
Katz E, Schran HF, Adashi EY.
A 37-year-old woman with a symptomatic 18-mm prolactin-secreting pituitary adenoma could not be managed with oral bromocriptine mesylate because of unacceptable gastrointestinal side effects. However, when given the medication intravaginally, the patient was successfully treated as assessed by evident tumor shrinkage and diminished secretory activity. Serum bromocriptine levels were approximately six to eight times higher than those reported after oral administration. The vaginal route may help reduce some of the adverse effects of bromocriptine mesylate experienced during oral administration and may possibly allow lowering of the overall effective dose by avoiding first liver passage.
Research on LSD and its derivatives led to drugs such as bromocriptine, which oppose the effects of histamine and estrogen. Some of bromocriptine’s effects are clearly antagonistic to serotonin, though bromocriptine is usually called a “dopamine agonist”; dopamine is pretty generally a serotonin antagonist. -Ray Peat, PhD
Eur J Pharmacol. 1982 Jul 30;81(4):569-76.
Actions of serotonin antagonists on dog coronary artery.
Brazenor RM, Angus JA.
Serotonin released from platelets may initiate coronary vasospasm in patients with variant angina. If this hypothesis is correct, serotonin antagonists without constrictor activity may be useful in this form of angina. We have investigated drugs classified as serotonin antagonists on dog circumflex coronary artery ring segments in vitro. Ergotamine, dihydroergotamine, bromocriptine, lisuride, ergometrine, ketanserin, trazodone, cyproheptadine and pizotifen caused non-competitive antagonism of serotonin concentration-response curves. In addition, ketanserin, trazodone, bromocriptine and pizotifen inhibited noradrenaline responses in concentrations similar to those required for serotonin antagonism. All drugs with the exception of ketanserin, cyproheptadine and pizotifen showed some degree of intrinsic constrictor activity. Methysergide antagonized responses to serotonin competitively but also constricted the coronary artery. The lack of a silent competitive serotonin antagonist precludes a definite characterization of coronary serotonin receptors at this time. However, the profile of activity observed for the antagonist drugs in the coronary artery differs from that seen in other vascular tissues. Of the drugs tested, ketanserin may be the most useful in variant angina since it is a potent 5HT antagonist, lacks agonist activity and has alpha-adrenoceptor blocking activity.
In an animal study, bromocriptine, which shifts the balance away from serotonin, reduced obesity and insulin and free fatty acids, and improved glucose tolerance. -Ray Peat, PhD
Neuroendocrinology. 1998 Jul;68(1):1-10.
Bromocriptine reduces obesity, glucose intolerance and extracellular monoamine metabolite levels in the ventromedial hypothalamus of Syrian hamsters.
Luo S, Meier AH, Cincotta AH.
We examined whether reductions in body fat stores and insulin resistance in Syrian hamsters induced by bromocriptine are associated with reductions in daily norepinephrine (NE) and serotonin activities as indicated by their extracellular metabolite levels in the ventromedial hypothalamus (VMH). High levels of these monoamines within the VMH have been suspected to induce obesity and insulin resistance. Microdialysate samples from the VMH of freely moving obese male hamsters (BW: 208 +/- 5 g) were collected hourly over a 25-hour period before bromocriptine treatment, during the first day of and after 2 weeks of bromocriptine treatment (800 microg/animal daily, i.p.), and body composition and glucose tolerance analyses were conducted before and after 2 weeks of treatments. The microdialysate samples were analyzed by HPLC for metabolites of serotonin: 5-hydroxy-indoleacetic acid (5-HIAA), NE: 3-methoxy-4-hydroxy-phenylglycol (MHPG), and dopamine: homovanillic acid (HVA). Bromocriptine treatment for 14 days significantly reduced body fat by 60% and areas under the glucose and insulin curves during a glucose tolerance test by 50 and 46%, respectively. Concurrently, extracellular VMH contents of 5-HIAA, MHPG, and HVA were reduced by 50, 29 and 66%, respectively (p < 0.05). Similarly, VMH 5-HIAA and MHPG contents were 48 and 44% less, respectively (p < 0.05), in naturally glucose-tolerant hamsters compared with naturally glucose-intolerant hamsters. Bromocriptine induced reductions of body fat, and improvements in glucose intolerance may result in part from its ability to decrease serotonin and NE activities in the VMH.
Metabolism. 1991 Jun;40(6):639-44.
Bromocriptine inhibits the seasonally occurring obesity, hyperinsulinemia, insulin resistance, and impaired glucose tolerance in the Syrian hamster, Mesocricetus auratus.
Cincotta AH, Schiller BC, Meier AH.
Seasonally obese-hyperinsulinemic female Syrian hamsters were injected daily with bromocriptine or saline for a period of 34 days to test for effects of bromocriptine on body fat store levels, hepatic triglyceride secretion, glucose tolerance, and plasma insulin and glucose concentrations. The effects of bromocriptine on body fat store levels, as well as on plasma insulin and glucose concentrations, in seasonally obese hamsters were compared with the levels of body fat, plasma insulin, and plasma glucose observed in seasonally lean hamsters. Bromocriptine treatment substantially improved glucose intolerance and reduced the total and stimulated areas under the glucose tolerance curve by 33% after 14 days of treatment. After 34 days of treatment, bromocriptine reduced body fat store levels by 36% and hepatic triglyceride secretion by 40% without any concurrent change in food consumption. Furthermore, bromocriptine reduced the plasma insulin level by 70%, while slightly reducing plasma glucose concentration (ie, 68% reduction in the insulin to glucose ratio). The reductions of body fat, plasma insulin, and plasma insulin to glucose ratio produced by bromocriptine in seasonally obese hamsters are equivalent to those observed in seasonally lean hamsters. Shifts in phase relationships of circadian neuroendocrine rhythms have been demonstrated to regulate annual cycles of metabolism in vertebrates, including the Syrian hamster. The effects of bromocriptine can also be explained as an alteration of such a circadian mechanism.
Metabolism. 1995 Oct;44(10):1349-55.
Bromocriptine inhibits in vivo free fatty acid oxidation and hepatic glucose output in seasonally obese hamsters (Mesocricetus auratus).
Cincotta AH, Meier AH.
Seasonally obese hyperinsulinemic hamsters were treated for 5 weeks with bromocriptine (500 to 600 micrograms per animal) and tested for drug effects on energy balance, body fat stores, nocturnal whole-body free fatty acid (FFA) metabolism and hepatic glucose output, and diurnal glucose tolerance. After 5 weeks, bromocriptine treatment reduced retroperitoneal fat pad weight by 45% without altering either daily food consumption or end-treatment total daily energy expenditure. Also, 5 weeks of treatment improved the diurnal glucose tolerance, resulting in a 47% and 33% decrease in the area under glucose and insulin curves, respectively. After 4 weeks, bromocriptine treatment reduced nocturnal lipolysis by 28%, palmitate rate of appearance into plasma by 30%, palmitate oxidation by 33%, and hepatic glucose output by 28%. Moreover, these reductions were accompanied by a 75% reduction in plasma insulin concentration. The data suggest that bromocriptine may improve diurnal glucose tolerance in part by inhibiting the preceding nocturnal lipolysis and FFA oxidation. Reductions in nocturnal FFA oxidation and hepatic glucose production may result from bromocriptine’s influences on circadian organization of hypothalamic centers known to regulate these activities. Available evidence suggests that bromocriptine may impact this neuroendocrine organization of metabolism by increasing the dopamine to noradrenaline activity ratio in central (hypothalamic) and peripheral (eg, liver and adipose) target tissues.
DIABETES CARE, VOLUME 20, NUMBER 11, NOVEMBER 1997
Effects of a Quick-Release Form of Bromocriptine (Ergoset) on Fasting and Postprandial Plasma Glucose, Insulin, Lipid, and Lipoprotein Concentrations in Obese Nondiabetic Hyperinsulinemic Women
Kamath and Associates
OBJECTIVE — To assess the effect on various aspects of carbohydrate and lipid metabolism of administering a quick-release formulation of bromocriptine (Ergoset) to obese, nondiabetic, hyperinsulinemic women.
RESEARCH DESIGN AND METHODS— Hourly concentrations of prolactin, glucose, insulin, free fatty acid (FFA), and triglyceride were measured for 24 h before and after approximately 8 weeks of treatment with Ergoset. In addition, fasting lipid and lipoprotein concentrations and the steady-state plasma glucose (SSPG) concentration in response to a continuous infusion of somatostatin, insulin, and glucose were determined before and after Ergoset administration.
RESULTS — Circulating prolactin concentrations were dramatically decreased (P < 0.001) following treatment, associated with a significant fall (P < 0.05) in 24-h-long plasma glucose, FFA, and triglyceride concentrations. Neither circulating plasma insulin concentrations nor the ability of insulin to mediate glucose disposal changed with treatment. Finally, fasting total cholesterol fell (P < 0.05) and the ratio of total to HDL cholesterol decreased (P = 0.06) in association with Ergoset treatment.
CONCLUSIONS — The fact that significant metabolic improvement was seen in the obese nondiabetic hyperinsulinemic women studied suggests that Ergoset could be of therapeutic benefit in clinical conditions of hyperglycemia and/or dyslipidemia.
J Hepatol. 2006 Sep;45(3):439-44. Epub 2006 May 6.
Bromocriptine reduces steatosis in obese rodent models.
Davis LM, Pei Z, Trush MA, Cheskin LJ, Contoreggi C, McCullough K, Watkins PA, Moran TH.
BACKGROUND/AIMS:
Obesity is a risk factor for glucose intolerance, steatosis, and oxidative stress, characteristics of nonalcoholic fatty liver disease. Bromocriptine may have anti-obesity, insulin-sensitizing, lipolytic, and antioxidant properties. We, therefore, hypothesized that bromocriptine would improve markers of nonalcoholic fatty liver disease in obese rodent models.
METHODS:
We performed a randomized, controlled experiment in genetically obese fatty Zucker rats and diet-induced obese rats to assess for behavioral and peripheral anti-obesity actions of bromocriptine (10mg/kg) that would improve nonalcoholic fatty liver disease.
RESULTS:
Behaviorally, food intake decreased and locomotor activity increased in bromocriptine-treated fatty Zucker and dietary-induced obese rats. Peripherally, liver triglycerides were significantly reduced and hepatic manganese superoxide dismutase significantly increased in bromocriptine-treated fatty Zucker and diet-induced obese rats compared to controls. Blood glucose was significantly lower in bromocriptine-treated Zucker rats compared to fatty controls and was no different than that of lean controls.
CONCLUSIONS:
Improvements in obesigenic behaviors, glucose tolerance, hepatic lipid accumulation, and mitochondrial oxidative stress observed in genetically obese and diet-induced obese rodents indicate that bromocriptine may be promising as a broad-based therapy for nonalcoholic fatty liver disease.
Eur J Pharmacol. 1984 Mar 16;99(1):85-90.
One minute of bromocriptine irreversibly inhibits prolactin release for hours.
Cronin MJ, Evans WS, Thorner MO.
The ability of the dopamine receptor antagonist spiperone to block dopamine- or bromocriptine-inhibited prolactin release from dispersed rat anterior pituitary cells was tested in vitro. In a continuously perfused cell column apparatus, spiperone rapidly counteracted the inhibitory effect of dopamine but was unable to reverse the inhibitory effect of the potent dopamine agonist bromocriptine when added 30 min after bromocriptine. However, spiperone completely blocked the bromocriptine action if added simultaneously with bromocriptine. These basic data were confirmed, and the time relationships more accurately defined, in static incubations of monolayer cultures. Spiperone blocked the typical inhibition of prolactin release by bromocriptine only if the cells were pre- or coincubated with the antagonist. If spiperone was added as soon as 1 min after bromocriptine, the antagonist was unable to block the complete expression of the bromocriptine inhibition. These results suggest that bromocriptine is a functionally irreversible dopamine agonist for at least the 4 h of these studies.
Proc Soc Exp Biol Med. 1984 Feb;175(2):191-5.
Bromocriptine inhibits growth hormone release from rat pituitary cells in primary culture.
Cronin MJ, Thorner MO, Hellmann P, Rogol AD.
The action of the potent dopamine receptor agonist bromocriptine was studied in primary cultures of rat anterior pituitary cells. Bromocriptine inhibited both prolactin and growth hormone release in a concentration-dependent manner. This effect was blocked by the dopamine receptor antagonist spiperone when the agonist and antagonist were added coincidently. In contrast, spiperone was unable to affect the actions of bromocriptine if added 1 min after bromocriptine application or later. These results suggest that dopamine receptors exist not only on mammotrophs, but also on somatotrophs in vitro.
J Neural Transm. 1981;51(1-2):61-82.
The endocrine profile of bromocriptine: its application in endocrine diseases.
Lancranjan I.
Bromocriptine, a potent agonist at Dz receptors, was developed as a therapeutic agent for inhibiting prolactin (PRL) secretion in patients with hyperprolactinemia. Besides, its PRL-suppressive effect and a short-lasting growth hormone (GH)-releasing effect in normal volunteers, bromocriptine has no other endocrine effects in healthy subjects. On the other hand, bromocriptine lowers GH secretion in acromegalic patients and ACTH secretion in some patients with Cushing’s disease or Nelson’s syndrome. The paper reviews the endocrine actions of bromocriptine in man, in normal and pathological conditions, the bromocriptine’s mechanism of action and its clinical applications in endocrinology.
Diabetes Care. 1996 Jun;19(6):667-70.
Bromocriptine (Ergoset) reduces body weight and improves glucose tolerance in obese subjects.
Cincotta AH, Meier AH.
OBJECTIVE:
A double-blind placebo controlled study investigated long-term effects of Ergoset, a new quick release formulation of bromocriptine, on body weight, body fat, and glucose tolerance in a group (n = 17) of obese subjects who were instructed to follow a moderate hypocaloric diet.
RESEARCH DESIGN AND METHODS:
Obese individuals (> 25% body fat for men and > 30% body fat for women) were instructed to follow a calorie-restricted diet (70% of weight maintaining based on study entry weight) and were randomized to daily treatment with Ergoset (1.6-2.4 mg/day) or placebo at 0800 over an 18-week treatment period. Oral glucose tolerance tests were performed on subjects before initiation and again at termination of treatment. Body weight and body fat (determined by skinfold measurements) were quantified every 2 weeks during the course of treatment.
RESULTS:
Ergoset treatment for 18 weeks significantly reduced body weight and body fat versus placebo (6.3 +/- 1.5 and 5.4 +/- 1.1 kg vs. 0.9 +/- 1.0 and 1.5 +/- 0.6 kg. respectively, P < 0.01). Ergoset, but not placebo, also improved glucose tolerance (P < 0.02); the stimulated area under the oral glucose tolerance curve was reduced by 46% (from 121 +/- 23 to 64 +/- 32 mg.h-1.dl-1), while the stimulated area under the insulin curve was reduced by 30%.
CONCLUSIONS:
When combined with instruction to follow a moderate hypocaloric diet, Ergoset, but not placebo, improves glucose tolerance and promotes significant weight and body fat loss in obese subjects over an 18- week treatment period.
Diabetes Care. 2000 Aug;23(8):1154-61.
Bromocriptine: a novel approach to the treatment of type 2 diabetes.
Pijl H, Ohashi S, Matsuda M, Miyazaki Y, Mahankali A, Kumar V, Pipek R, Iozzo P, Lancaster JL, Cincotta AH, DeFronzo RA.
OBJECTIVE:
In vertebrates, body fat stores and insulin action are controlled by the temporal interaction of circadian neuroendocrine oscillations. Bromocriptine modulates neurotransmitter action in the brain and has been shown to improve glucose tolerance and insulin resistance in animal models of obesity and diabetes. We studied the effect of a quick-release bromocriptine formulation on glucose homeostasis and insulin sensitivity in obese type 2 diabetic subjects.
RESEARCH DESIGN AND METHODS:
There were 22 obese subjects with type 2 diabetes randomized to receive a quick-release formulation of bromocriptine (n = 15) or placebo (n = 7) in a 16-week double-blind study. Subjects were prescribed a weight-maintaining diet to exclude any effect of changes in body weight on the primary outcome measurements. Fasting plasma glucose concentration and HbA(1c) were measured at 2- to 4-week intervals during treatment. Body composition (underwater weighing), body fat distribution (magnetic resonance imaging), oral glucose tolerance (oral glucose tolerance test [OGTT]), insulin-mediated glucose disposal, and endogenous glucose production (2-step euglycemic insulin clamp, 40 and 160 mU x min(-1) x m(-2)) were measured before and after treatment.
RESULTS:
No changes in body weight or body composition occurred during the study in either placebo- or bromocriptine-treated subjects. Bromocriptine significantly reduced HbA(1c) (from 8.7 to 8.1%, P = 0.009) and fasting plasma glucose (from 190 to 172 mg/dl, P = 0.02) levels, whereas these variables increased during placebo treatment (from 8.5 to 9.1%, NS, and from 187 to 223 mg/dl, P = 0.02, respectively). The differences in HbA(1c) (delta = 1.2%, P = 0.01) and fasting glucose (delta = 54 mg/dl, P < 0.001) levels between the bromocriptine and placebo group at 16 weeks were highly significant. The mean plasma glucose concentration during OGTT was significantly reduced by bromocriptine (from 294 to 272 mg/dl, P = 0.005), whereas it increased in the placebo group. No change in glucose disposal occurred during the first step of the insulin clamp in either the bromocriptine- or placebo-treated group. During the second insulin clamp step, bromocriptine improved total glucose disposal from 6.8 to 8.4 mg x min(-1) kg(-1) fat-free mass (FFM) (P = 0.01) and nonoxidative glucose disposal from 3.3 to 4.3 mg min(-1) x kg(-1) FFM (P < 0.05), whereas both of these variables deteriorated significantly (P < or = 0.02) in the placebo group.
CONCLUSIONS:
Bromocriptine improves glycemic control and glucose tolerance in obese type 2 diabetic patients. Both reductions in fasting and postprandial plasma glucose levels appear to contribute to the improvement in glucose tolerance. The bromocriptine-induced improvement in glycemic control is associated with enhanced maximally stimulated insulin-mediated glucose disposal.
Acta Neurol Scand. 1977 Jul;56(1):37-45.
Treatment of Huntington’s chorea with bromocriptine.
Frattola L, Albiazzati MG, Spano PF, Trabucchi M.
The authors tested the effects of 2-Br-ergocriptine (bromocriptine, CB-154), a drug which exerts a mixed agonist-antagonist activity on the dopaminergic receptors, in 12 patients with Huntington Chorea in a double-blind crossover trial. This treatment significantly reduced the abnormal involuntary movements and the disease severity in most of the patients. Subjects who were slightly disabled showed a better response than the ones with more severe degrees of disability.
Obstet Gynecol. 1980 Mar;55(3):278-84.
Bromocriptine mesylate (Parlodel) in the management of amenorrhea/galactorrhea associated with hyperprolactinemia.
Cuellar FG.
The efficacy and safety of bromocriptine mesylate (5 to 7.5 mg per day for up to 24 weeks) were studied in 22 clinical trials involving 226 patients who had amenorrhea/galactorrhea associated with hyperprolactinemia and no demonstrable pituitary tumor. Of the 187 patients evaluated for efficacy, 80% experienced reinitiation of menses (or pregnancy without first having menses); the average treatment time (excluding those who conceived) was 5.7 weeks. Galactorrhea was significantly (at least 75%) reduced in 76% of the 187 patients after an average treatment tome of 6.4 weeks, and was completely suppressed in 58% after 12.7 weeks. Maximum reduction in serum prolactin levels occurred within the first 4 weeks of therapy and the reduced levels were maintained during treatment; moreover, there was a strong correlation between prolactin reduction and clinical improvement. Adverse reactions were reported by 68% of the 226 patients evaluated for safety; in general, these reactions were mild and transient. Several patients experienced hypotension, but only 1 discontinued therapy because of it. Based on these findings, bromocriptine mesylate was judged safe and efficacious for this purpose.